Bilingual and Cross-Cultural Professional Company
We Provide Turn-Key Solutions for MedTech Clients Enter into China Market and Keep Life-Cycle China Regulatory Compliance
100+
Products Registered
130+
Happy Clients
2
Regional Offices
45+
RA/QA Consultants and In-net experts
Our professional services are focused on the end-to-end Regulatory affairs Issues. Our bilingual and cross-cultural experts help you with the regulation’s interpretation in a manner of US FDA terms as well as introduction of the background behind the scenes. We provide you hands-on regulatory compliance solutions to release you from the frustration.
Medical Device Registration in China
Our service covers the overall strategy, specific concerns, type testing, to submission dossier preparation.
GMP Compliance
Our service covers the life-cycle compliance from pre-approval to post-market. We also provide tailored China GMP training and QMS GAP analysis.
Clinical Evaluation
Our Service covers the clinical pathway strategy report, clinical evaluation report (CER) , and clinical trial coordination.
Post Market Surveillance
Medical device post market surveillance includes annual report (AR), product recall, adverse events reporting (AE) and periodical re-evaluation of the risks (PRER).
Added-Value Services
We dedicated to providing you added-value services on-line, or in person.
Webinars
Newsletters
Blogs
On-line Meetings
Served Products
The medical device industry is a multidisciplinary field. We are proud of our involvement in the product area thanks to the efforts of our team of experts and external in-net consultants. The service includes product registration, Clinical evaluation, Whole Life-Cycle compliance, GMP Training, Supplier Audit, etc.
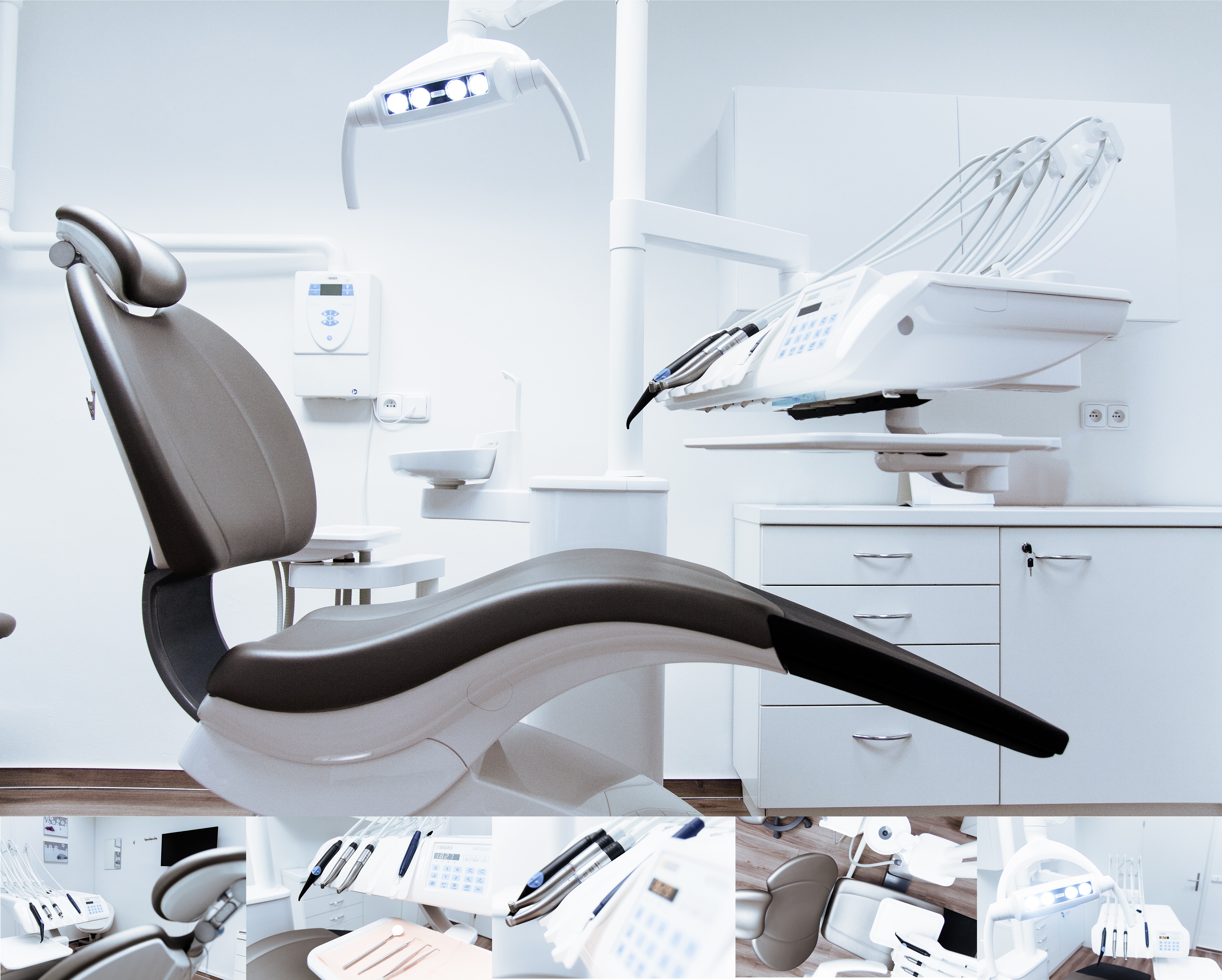
Implantable Sterile Products
Coronary drug-eluting stent Aortic valve, prosthesis, percutaneously deliver System
Energy-based Aesthetic Devices
Laser-based Devices Radiofrequency (RF)-based Devices Light-based Devices Ultrasound Devices
Respiratory Devices
Nebulizer Respiratory humidifier
Assisted Reproductive Device
Oocyte Collection Needle Intracytoplasmic Sperm Device
IVD
Immunoassay Reagents and Kits
Orthopedic Diagnostics Device
Endoscopic Release System Arthroscopy System
How It Works
Hands-on service is our promise to the clients. You won’t feel overwhelmed by not understanding Chinese culture and customs. It’s on us.
Once we receive inquiries from our clients, we will offer 15 min free consultation to understand clients’ demand. We will not start until you think our services match your needs. After signing the agreement, we will communicate with you to build up an overall plan, and set up the milestones. Report will be sent to you by milestone or per your request.
Confidential Agreement
We respect our clients' intellectual property rights and trade secrets. We will sign a confidentiality agreement before we officially cooperate.
Client Side
Provide documents and samples according to our list and the confirmed timeline. periodically meet with us to align our understanding Review the documents prepared by us and confirm its accuracy.

GVM Side
Answer your questions or concerns. Interpret relevant regulatory provisions and context. Identify relevant product standards and guidance documents. Coordinate the testing, clinical trial. Finish the report or submission dossier according to project timeline. Communicate with reviewer, test institute and NMPA officials. Other tasks defined by the agreement.
Office Locations
Our BD and PM teams are based in US to serve our MedTech clients in a timely manner. Our RA/QA professional team is based in China to leverage the local resources and network, and keep great comminication with regulatory agency, both NMPA and local branches
Guangzhou, China
1701 Kingold Century Centre, No.62, Jinsui Road, Tianhe District, Guangzhou, P.R. China 510623
